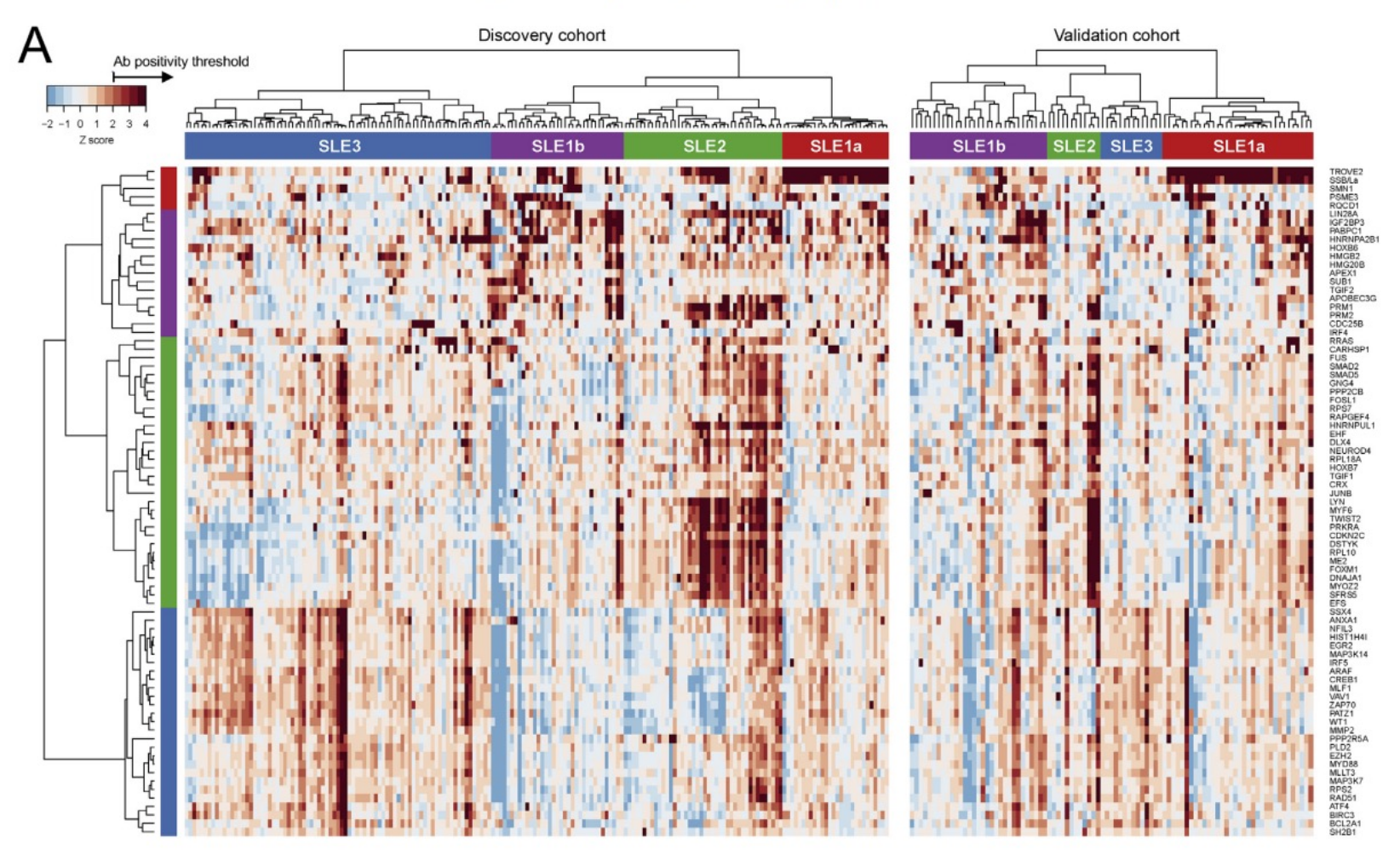
Autoantibody signatures can be used to identify disease subtypes. This can lead to a better understanding of the underlying pathogenic mechanisms, informing drug discovery and patient treatment decisions.
Predictive autoantibody biomarker signatures can be developed to identify drug responders and non-responders, and biologically relevant sub-cohorts. These insights enable intra-disease stratification, driving more efficient and economical study designs and clinical trial patient enrichment.
Having the ability to interrogate many autoantibody targets in a single assay can help accelerate the identification and validation of signatures with high predictive value.